CHAPTER
|
VINYL POLYMERIZATION IN AQUEOUS MEDIA |
2.1 INTRODUCTION
Polymerization of vinyl monomers in aqueous media may be classified into 4 different systems.
1.
Solution polymerization
Both monomer and formed polymer are soluble in water
2.
Precipitation polymerization
Monomer is soluble in water and polymer is not soluble in water.
3.
Emulsion polymerization
Both monomer and polymer are insoluble in water. Monomer is emulsified by
emulsifiers in water.
4.
Suspension polymerization.
Both monomer and polymer are insoluble in water. Monomer is dispersed as
droplets in water by the effect of a stirrer and suspension stabilizer is used.
Polymerization of a vinyl monomer in a solvent overcomes many of disadvantages of the bulk polymerization process. The solvent acts as diluent and aids in the transfer of the heat of polymerization. The solvent also allows easier stirring, since the viscosity of the reaction mixture decreased. On the other hand, the presence of solvent may present new difficulties. Chain transfer to solvent can become a problem. Vinyl acetate, acrylonitrile, ester of acrylic acid are polymerized in solution.
When water is used as solvent, there are some advantages over organic solvents. Water is a cheap, clean and environmental friendly solvent and chain transfer to solvent is not present. However, for homogenous polymerization process it is limited to only water-soluble polymers and monomers.
Industrially important water soluble polymers are polyacrylamide, poly (N-vinyl pyrrolidone) and polyacrylic acid.
In industry, the water-soluble polymers are recovered from their water solutions by spray drying. In laboratory, polymers are precipitated by pouring their water solution into non-solvents such as acetone and methanol.
2.2 INITIATORS
Water-soluble azo initiators peroxydisulfate and redox systems of peroxy compound and reducing agent are the main water soluble initiators.
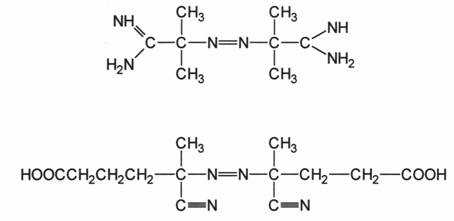
2.2.1 WATER-SOLUBLE AZO INITIATORS:

2.2.2 PEROXVDISULFATES
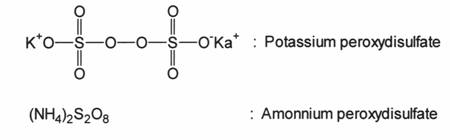
2.2.3 REDOX SYSTEMS
|
Oxidizing Agent |
Reducing Agent |
|
H2O2 |
Ag+, Fe2+, Ti3+ |
|
K2S2O3 |
S2O32-, S3O52- |
2.3 REACTION MECHANISMS
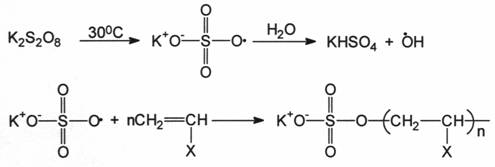
2.3.1 PEROXYDISULFITE
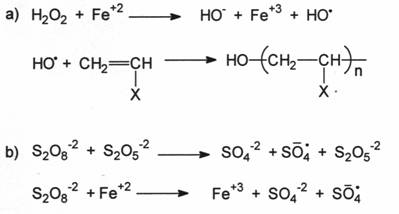
2.3.2 REDOX SYSTEMS
2.4.1 EXPERIMENT I
In a 500 ml flask equipped with a stirrer and gas inlet and outlet tube, are dissolved 2.5 g of pure acrylamide in 250 ml distilled water nitrogen is bubbled through this solution for about 10 minutes.
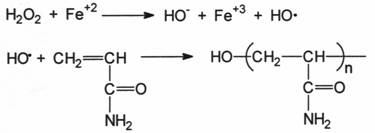
To this solution arc added 12.5 ml of 0.1 molar aqueous solutions of ferrous ammonium sulfate and 25 ml a 0.1 molar solution of hydrogen peroxide. The polymerization is carried out at room temperature. After about 1/2 h, the viscous solution is obtained. This solution is added dropwise with vigorous stirring to 2 I methanol containing a few drops of hydrochloric acid.
2.4.2 EXPERIMENT II
Polymerization of methacrvlic acid with potassium peroxydisullate in aqueous solution. In a 250 ml three-necked flask equipped with reflux condenser and two dropping funnels 55 ml of distilled water are heated to 80°C. At this temperature, 15g methacrylic acid purified by vacuum distillation under nitrogen and a solution of 0.4g potassium peroxydisulfate in 8 ml of water are introduced dropwise into the flask under slow agitation with a magnetic stirrer. The Methacrylic acid polymerizes immediately as is evident from the increase in viscosity of the solution. A after the reagents have been added, the temperature of reaction mixture is maintained at 80°C for another hour. The viscous solution is mixed with a mixture of 140 ml acetone and 40 ml water, filtered and then added dropwise 1 L of a 4: 1 mixture of acetone and petrol ether (50/70). The polymer precipitates and the supernatant liquid is decanted and 500 ml of acetone: petrol ether (4: 1) is added. The polymer is filtered and extracted with petrol ether for 5 hours and subsequently is dried in vacuum at 50 "C.
2.4.2 EXPERIMENT III
Into 250 ml round-bottomed flask 175 ml water is added and nitrogen is babbled for about 1/2 h. Than 15 ml acrylonitrile, 0.5 ml of Na2S2O5 solution (5% in water) and 2.5 ml of FeSO4.7H2O, solution (10 mg in 100 ml water+2 ml conc. H2S04) are introduced and subsequently 2.5 ml of K2S2O8 solution (5% in water) is added and mixed briefly at 20°C. The mixture is stirred at 20°C for about 1 h, the precipitated polymer is filtered washed with water then with methanol and dried at 50°C in vacuum.