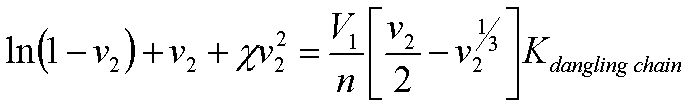
Calculation of Swelling for a Gel
The Flory-Rehner Equation (Flory's first book) describes swelling of network polymers as a function of the enthalpic interaction parameter between the polymer unit and the solvent, c, the molar volume of the solvent, V1, and the molecular weight between crosslink sites, n.
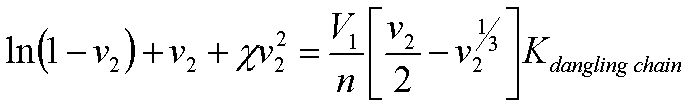
where v2 is the volume fraction of polymer in the swollen gel [Flory's first book page 579 eqn. 39']. The swelling ratio, Q, is given by 1/v2. Equation (1) predicts that the swelling ratio, Q, increases dramatically with molecular weight between entanglements and with the negative value of the interaction parameter. If we ignor the entropic part of equation (1), first 2 terms, and take the larger term in the brackets on the right side (remember v2 is < 1), and use Q = 1/v2, we find as a rule of thumb that swelling increases with negativity of c and with n,
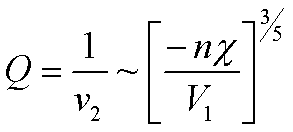
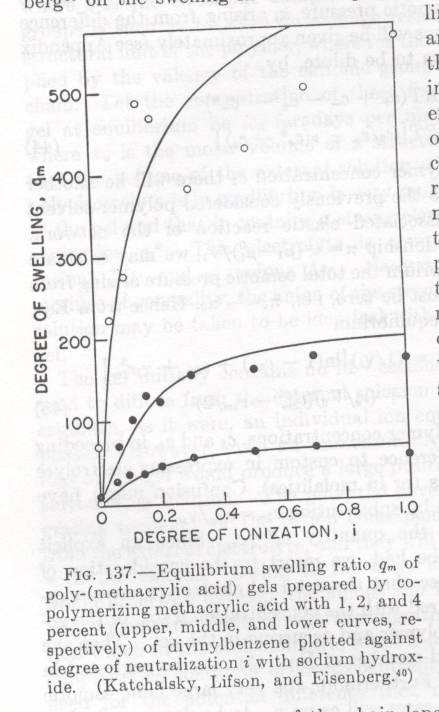
Figure 1. Swelling ratio for ionic gels as a function of degree of ionization (0 to 1) and crosslink density or n.
Figure 1 shows the behavior of equation (1) compared to experimental data from polymethacrylic acid gels (sodium salts) crosslinked using divinyl benzene. n drops with larger percentage divinylbenzene. The interaction parameter becomes more negative with higher degree of ionization. Notice the degree of swelling can reach more than 5 times. In commercial hydrogels the degree of swelling can reach 5,000 to 10,000 or higher (50 to 100x swelling).