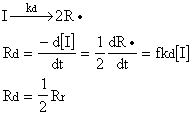
From http://www.fbe.itu.edu.tr/polymer/lab/Exp1.dot ISTANBUL Turkey
CHAPTER
|
SYNTHESIS OF MACROMOLECULAR SUBSTANCES BY FREE RADICALPOLYMERIZATION MECHANISM |
1.1 INTRODUCTION
Polymerization reactions can proceed by various mechanisms and be catalyzed by initiators. For addition polymerization of single compounds, initiation of chains may occur via radical, cationic, anionic or coordinate-acting initiators, but some monomers will not polymerize by more than one mechanism.
1.2 FREE RADICAL HOMO POLYMERIZATION
Free radical polymerization are initiated by radicals and propagated by macroradicals. These radicals exhibit an unpaired electron.
Initiating radicals are rarely formed by monomers themselves but rather thermally, electrochemically or photo-chemically from deliberately added initiators.
Initiation in a free-radical polymerization consists of two steps: 1) A dissociation of the initiator to form radical species, 2) Addition of a single monomer molecule to the initiating radical (=association step).
The rate of dissociation of initiators, I, usually follows 1st order kinetics and is dependent upon the solvent present and the temperature of polymerization of polymerization as well.
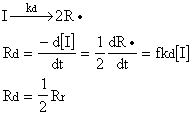
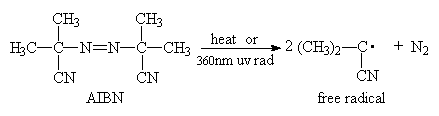
Some common thermal initiators are: 2,2’-azo-bis-isobutyronitrile (AIBN); t-butyl hydroperoxide; cumyl peroxides; benzoyl peroxide (BPO); t-butyl peroxide; lauroyl peroxide, dipotassium persulfate.
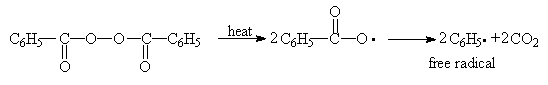
The initiator efficiency, f, is seldom 100 %, since the recombination and other side reactions of the free radicals can occur during the initiation reaction. Besides thermally decomposing initiators photo and redox initiators are also well known.
In the association step of initiation:
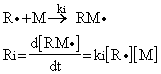
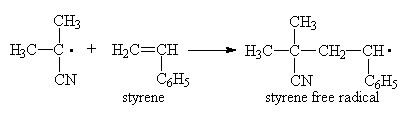
Where ki is the rate constant for monomer association.
After the initiation reactions, additional monomer units are added to the initiated monomer species as:
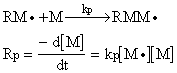
This step is called propagation and kp is the propagation rate constant.
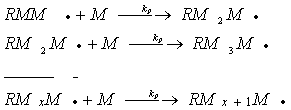
![]()
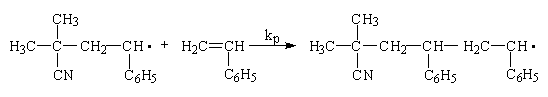
Additional monomers are added sequentially during subsequent propagation steps. Propagation will continue until some termination process occurs. Termination can occur by combination, by disproportionation or by chain transfer reactions. Termination by combination occurs when two propagating radical chains of arbitrary degrees of polymerization meet at their free-radical ends.
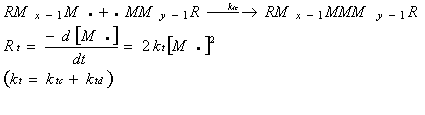
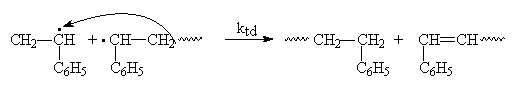
Termination by disproportionation gives two terminated chains. In this case, one terminated chain will have an unsaturated carbon group while the other terminated end is saturated.
![]()
Termination by chain transfer can be achieved by hydrogen abstraction from an initiator, monomer, polymer or solvent molecule.
![]()
The radical site is transferred to the chain-transfer agent (S·) to continue polymerization process. The number-average degree of polymerization decreases if transfer reactions show strongly their presence.
1.3 IDEAL KINETICS
Ideal polymerization kinetics assumes:
According to these assumptions, one can write, Ri=Rr where Rr =2Rd. Because, initiator radicals are consumed as fast as they generated.
![]()
In the steady state (Bodenstein steady state) principle, as many radicals are formed as disappear by termination.
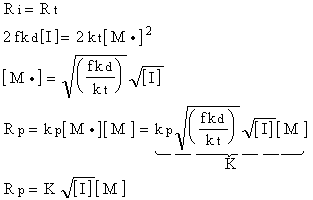
This shows that the polymerization rate in free radical polymerization is proportional to monomer concentration and to the square root of initiator concentration.
In that case, we neglect that thermally initiation of monomer. If this case is valid,
![]()
Must be written. On the other hand,
![]()
is true for considering the thermal polymerization of monomers.
If the conversion is small (at the beginning of polymerization).
![]()
![]() can
be written. That is,
can
be written. That is,
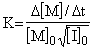 or
or
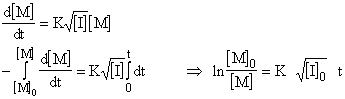
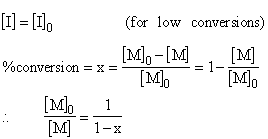
![]()
For less than 15 % conversions, if you have a data of percent conversion changing with time at a constant initiator concentration, overall rate constant K can be calculated for a constant temperature:
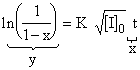
1.4 KINETIC CHAIN LENGTH AND THE DEGREES OF POLYMERIZATION
The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic chain length.
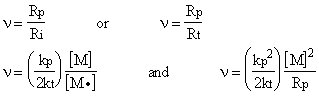
can be written.
The kinetic chain length is inversely proportional to the radical concentration and therefore to the rate of polymerization. If the k p2 / k t ratio is given, n can be calculated.
For termination reaction by combination of radicals, each polymer molecule forms in a mono-radical-initiated polymerization consisting of two kinetic chains grown from unrelated primary radicals. This idea gives that:
![]()
If termination occurs by
disproportionation, ![]() is
also proportional to
n
but the constant of proportionality lies between one and two, depending on the
fraction of termination, which occurs by combination.
is
also proportional to
n
but the constant of proportionality lies between one and two, depending on the
fraction of termination, which occurs by combination.
Total number of polymer molecules may be equal to the number of pairs of chain ends, and the average degree of polymerization is the ratio of the total number of monomers polymerized to this number of pairs. The average degree of polymerization of the polymer formed in any small time interval will be given by the ratio of the rate of polymerization (Rp) to the rate of production of pairs of chain ends.
Two macroradicals may terminate by combination or chain transfer of a hydrogen atom at one chain end to a free radical end of another chain in a chain-transfer process. A reaction in which the free radical center is somehow transferred from a growing chain to another molecule to terminate the growing of the chain while obtaining a new radical being capable of starting a new chain is known chain transfer reaction. The molecule, which takes part in the chain transfer reaction, is called (chain) transfer agent.
Solvent SH may react with the growing radical as follows:
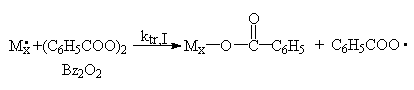
The initiator may also function as a transfer agent:
![]()
The monomer is also considered a transfer agent
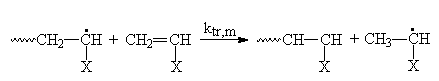
(b carbon atom of the radical chain may be transferred to the unsaturated monomer.)
The free radicals produced by chain transfer may or may not initiate another polymer chain formation on its activity.
If all of the kinetic chains start from monoradicals released by an initiator, the reciprocal of degree of polymerization:

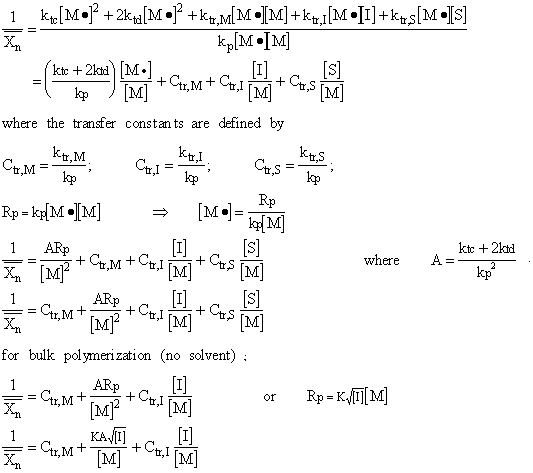
The intercept of the plot gives Ctr,M.
For experimental determination of the transfer constants, the monomer concentration must be kept constant. Therefore, the conversion of monomers to polymer must be less than 10 %.
1.5 EXPERIMENTS
1.5.1 MATERIALS REQUIRED
1.5.1.1 Apparatus: Vacuum distillation system, Pyrex freeze & thaw system, 5 Pyrex tubes having a ground joint and attachable stopcock, Dewar flasks, liquid nitrogen, sintered glass crucible (No: 2 porosity), thermostated oil bath (for 800C), viscometer, chronometer, water bath (for 250C).
1.5.1.2 Chemicals: styrene, NaOH pellets, calcium chloride 2,2’-azo-bis-isobutyronitrile (AIBN), toluene, methanol.
1.5.2 EXPERIMENTAL PROCEDURE
1.5.2.1 Bulk polymerization of styrene with 2,2’-azobisisobutyronitrile (AIBN)
Monomeric styrene is freed from inhibitors by shaking twice with 10 % sodium hydroxide solution, washing three times with distilled water, drying over calcium chloride or silica gel and distilling under reduced pressure (b.p. 820C / 100 torr, 460C / 20 torr). It is stored in a refrigerator until required.
4 Pyrex tubes, having a ground joint and attachable stopcock, are charged with 7.4 mg (0.045 mmol), 14.7 mg (0.098 mmol), 22.1 mg (0.13 mmol), and 29.5 mg (0.12 mmol) of (AIBN), respectively, together with 4 g (38.4 mmol) of destabilized styrene; 4 g of styrene only are weighed into a fifth tube. The AIBN is dissolved in the styrene by shaking.
The tubes are now cooled in liquid nitrogen cold bath, thereby freezing the styrene (m.p.-30.6 oC); after evacuation with a filter pump and thawing, the tubes are filled with nitrogen. This sequence is repeated twice more. Finally the tubes are sealed off.
The samples are polymerized at 80o C by placing in an appropriately adjusted thermostat for 20 minutes them rapidly cooled in ice-water.
The mixture in the tubes are dissolved in toluene and precipitated in methanol. The filtered polymers are washed with methanol twice and filtered off using sintered glass crucibles and dried to constant weight in vacuum at 50o C. The results are plotted as rate of polymerization (% conversion) against the square root of the initiator concentration (in mole %), and as degree of polymerization against the reciprocal of the square root of initiator concentration (g=0.851 g/cc at 80o C for styrene).
Molecular weights of the samples are determined by using viscometric measurements. The efflux time for toluene (to) and for five different concentrations of each polystyrene samples (t) in toluene are measured at 25oC. The limiting viscosity numbers [h] of all samples are determined from the intercepts of the
plots of
h
sp/c vs. c, where ![]()
The molecular weights of samples are calculated by using the following equation:
[h] = 4.4 x 10-4 Mv 0.65
The rate of polymerization (in % conversion) is plotted against the square root of the initiator concentration to calculate the overall rate constant and the degree of polymerization is plotted against the reciprocal square root of the initiator concentration to calculate the monomer transfer constant.
REFERENCES
1. R.B. Seymour, C.E. Carraher Jr., ”An Introduction to Polymer Chemistry”, Marcel Dekker Inc. 2nd Ed. New York, 1988.
2. H.G. Elias, “ An Introduction to Polymer Science”, Verlagsgesellschaft mbh, Weinheim, 1997
3. D. Braun, H. Cherdron, and W. Kern “Practical Macromolecular Organic Chemistry” Vol.2 Harwood Academic Publishers, 3rd Rev. Ed., New York, 1984.