 Figure
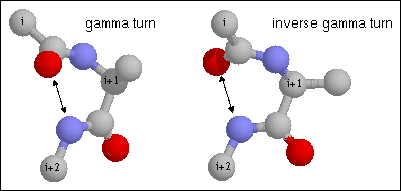
14. Gamma turn. Note the hydrogen bond between CO of residue i and NH of
residue i+2. The dihedral angles of residue i+1 are (70, -60) and (-70, 60) for
phi and psi of the classical and inverse gamma turns. (from http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
Figure
14. Gamma turn. Note the hydrogen bond between CO of residue i and NH of
residue i+2. The dihedral angles of residue i+1 are (70, -60) and (-70, 60) for
phi and psi of the classical and inverse gamma turns. (from http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
 Figure
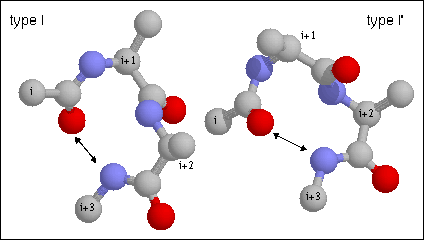
15. Type I turn. Note the hydrogen bond between CO of residue i and NH of
residue i+3. The backbone dihedral angles of residue are (-60, -30) and (-90, 0)
of residues i+1 and i+2, respectively of the type I turn. Proline is often
found in position i+1 in type I turns as its phi angle is restricted to -60 and
its imide nitrogen does not require a hydrogen bond. Glycine is favored in this
position in the type II' as it requires a positive (left-handed) phi value.
(from
http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
Figure
15. Type I turn. Note the hydrogen bond between CO of residue i and NH of
residue i+3. The backbone dihedral angles of residue are (-60, -30) and (-90, 0)
of residues i+1 and i+2, respectively of the type I turn. Proline is often
found in position i+1 in type I turns as its phi angle is restricted to -60 and
its imide nitrogen does not require a hydrogen bond. Glycine is favored in this
position in the type II' as it requires a positive (left-handed) phi value.
(from
http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
 Figure
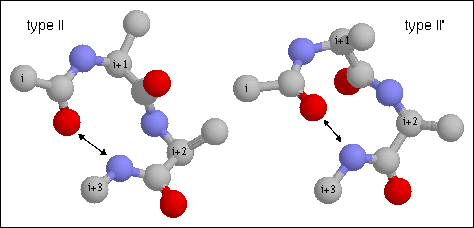
16. Type II turn. Note the hydrogen bond between CO of residue i and NH of
residue i+3. The backbone dihedral angles of residue are (-60, 120) and (80, 0)
of residues i+1 and i+2, respectively of the type II turn. Proline is often
found in position i+1 in type I turns as its phi angle is restricted to -60 and
its imide nitrogen does not require a hydrogen bond. Glycine is favored in this
position in the type II' as it requires a positive (left-handed) phi value.
(from http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
Figure
16. Type II turn. Note the hydrogen bond between CO of residue i and NH of
residue i+3. The backbone dihedral angles of residue are (-60, 120) and (80, 0)
of residues i+1 and i+2, respectively of the type II turn. Proline is often
found in position i+1 in type I turns as its phi angle is restricted to -60 and
its imide nitrogen does not require a hydrogen bond. Glycine is favored in this
position in the type II' as it requires a positive (left-handed) phi value.
(from http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
 3.10
Helix
3.10
Helix
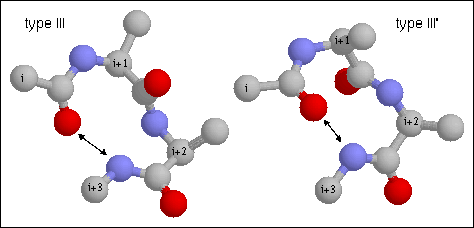
Figure 17. Type III turn. Note the hydrogen bond between CO of residue i and
NH of residue i+3. This is a single turn of right-handed (III) and left-handed
(III') 3.10 helix, respectively. The backbone dihedral angles of residue are
(-60, -30) and (-60, -30) of residues i+1 and i+2, respectively of the classical
type III turn. (from http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
 Figure
14. Gamma turn. Note the hydrogen bond between CO of residue i and NH of
residue i+2. The dihedral angles of residue i+1 are (70, -60) and (-70, 60) for
phi and psi of the classical and inverse gamma turns. (from http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
Figure
14. Gamma turn. Note the hydrogen bond between CO of residue i and NH of
residue i+2. The dihedral angles of residue i+1 are (70, -60) and (-70, 60) for
phi and psi of the classical and inverse gamma turns. (from http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15) Figure
15. Type I turn. Note the hydrogen bond between CO of residue i and NH of
residue i+3. The backbone dihedral angles of residue are (-60, -30) and (-90, 0)
of residues i+1 and i+2, respectively of the type I turn. Proline is often
found in position i+1 in type I turns as its phi angle is restricted to -60 and
its imide nitrogen does not require a hydrogen bond. Glycine is favored in this
position in the type II' as it requires a positive (left-handed) phi value.
(from
http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)
Figure
15. Type I turn. Note the hydrogen bond between CO of residue i and NH of
residue i+3. The backbone dihedral angles of residue are (-60, -30) and (-90, 0)
of residues i+1 and i+2, respectively of the type I turn. Proline is often
found in position i+1 in type I turns as its phi angle is restricted to -60 and
its imide nitrogen does not require a hydrogen bond. Glycine is favored in this
position in the type II' as it requires a positive (left-handed) phi value.
(from
http://www.cryst.bbk.ac.uk/PPS2/course/section8/ss-960531_16.html#HEADING15)