EXPERIMENT 9
ZIEGLER-NATTA POLYMERIZATION OF ETHYLENE
Introduction
The catalytic properties of ill defined, heterogeneous organometallic mixtures with respect to polymerization of simple olefins (i.e. ethylene or propylene) were announced by K. Ziegler and G. Natta in 1955. Ziegler's original discovery may be summarized in eq-1:
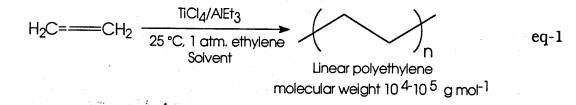
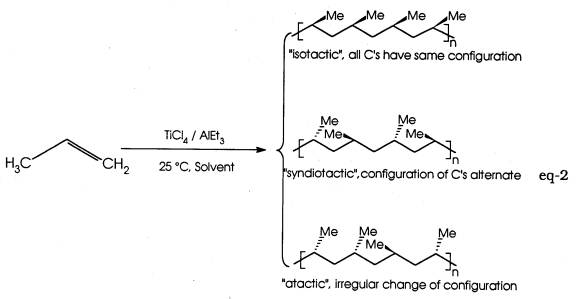
Natta's contribution was to apply the Ziegler catalyst to propylene and establish a connection between the stereochemistry of the polymer so formed and its bulk properties (eq-2). Both from a fundamental and technological viewpoint these discoveries was quite profound, and in recognition of their work both scientists were awarded the Nobel Prize in 1963.
While the mechanism of this multi C-C bond formation reaction is still controversial, in industry some millions of tons of polyethylene and polypropylene are synthesized by these simple Ziegler catalysts (i.e. titanium salts and aluminum alkyls) and their derivatives annually. The ease of synthesis and utility of these polymers are the reasons why plastics are so ubiquitous in modern life. In this experiment a basic Ziegler-Natta catalyst will be used to catalyze the synthesis of polyethylene.
Outline
A slurry of Ziegler-Natta catalyst is prepared from AlEt3 and TiCl4 in hexane; this is then used to polymerize ethylene gas to solid polyethylene.
Precautions
Both TiCl4 and AlEt3 are corrosive, so gloves (and of course glasses) must be worn while they are being transferred. These reagents are provided as dilute solutions in petroleum to reduce their reactivity.1 Any spillages of TiCl4 or AlEt3 solutions should be covered with some dry sand or vermiculite - DO NOT USE WATER!
Procedure - Period 1
Inert Atmosphere Box
Titanium Tetrachloride and Triethylaluminium are stored in the inert atmosphere/dry box because of their reactivity towards oxygen and water. The first part of this experiment involves measuring out the required volumes of these reagents and sealing them in glass vials before removing them from the box.
Check the needles and syringes provided to ensure that they are clean and dry, and that the needles are not blocked; put them in the oven (about 100°C) to dehydrate their surfaces.
1.Obtain two 6 dram (approx. 23 ml) vials from the oven and allow them to cool, label one as Ti(Cl)4 and the other as Al(Et)3, together with your initials and the date.
2. Place syringes, needles, vials and two B14 septums in the thin tray provided (all disassembled so that air can be pumped away).Then place tray in the mini antechamber of the glovebox and secure outer door.
3. Evacuate and refill the antechamber with N2 at least three times before opening the inner door to the glove box.
Each student should use the gloves to fill one of the vials.
4. Syringe 20mL of each stock solution into each vial. Use the lowest numbered stock solution with a hole in the cap.
5. Push a B14 septum on to each vial and place the vials on a shelf until the next period if the experiment will not be concluded the same day.
Prepare beakers of the 3 syringe washing solutions described below.
6. Place needles, syringes and vials of reagents in the tray and place tray in the mini antechamber. Close inner door securely. With tap in middle position open outer door and remove contents.
Immediately afterwards
wash the syringes with Solution #1 (i.e.
the petroleum ether)
then wash it with Solution #2, (i.e. the mixture
of ethanol and acetic acid)
wash it with 0.1 M HCl solution
then with
copious water,
finally, disassemble it and wash the pieces with ethanol and
place in oven.
Polymerization of Polyethylene
1.In a fume cupboard with a nitrogen supply and an ethylene cylinder
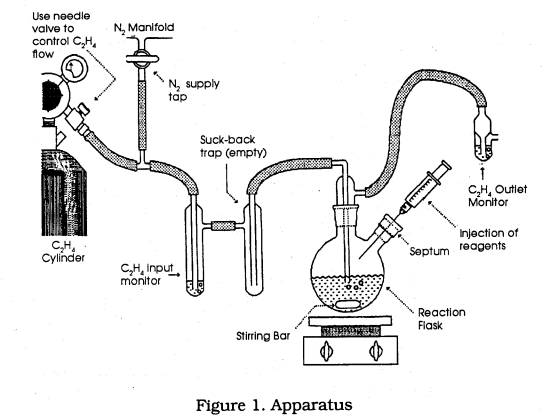
* assemble the apparatus shown in Figure 1.
* Inspect each piece to ensure that it is dry.
* Commence flushing the apparatus with a generous nitrogen flow.
* Flush some nitrogen back up the ethylene hose by momentarily disconnecting the hose from the ethylene cylinder nozzle.
UNDER
NO CIRCUMSTANCES SHOULD A COMPRESSED GAS CYLINDER (any variety!!) BE
"DEAD-ENDED"
(What does this mean?)!!
 <![endif]>
<![endif]>2. Meanwhile, remove the syringes and needles from the oven, assemble them hot, and allow them to cool. Check that the needles are screwed firmly into the syringes' Luer locks, but beware of stripping the threads.
3. Obtain from the experiment locker a container of dry sand or vermiculite and from the glove-box the solutions of TiCl4 and AlEt3 in hexanes. Connect a needle to a syringe outlet from the nitrogen manifold.
4. In the presence of a demonstrator, insert the nitrogen needle into the septum of the vial. Ensure the nitrogen needle does not dip down into the solution - this avoids the possibility of solution finding its way back up into the manifold (a messy situation!).
5. Make ready two small containers (e.g.. beakers) of syringe washing liquids:- (Solution #1) petroleum ether of any kind (about 20ml) and (Solution #2) a mixture of ethanol (20ml) and acetic acid (5ml).
6.Flush a syringe with nitrogen and transfer some AlEt3 stock solution (20ml) into the reaction flask.
Immediately
afterwards
wash the syringe with Solution #1 (i.e. the petroleum
ether)
then wash it with Solution #2, (i.e. the mixture of ethanol
and acetic acid)
wash it with 0.1 M HCl solution
then with copious
water,
finally, disassemble it and wash the pieces with ethanol and place
in oven.
7. Remove the nitrogen needle from the AlEt3 vial. Begin stirring the AlEt3 in the reaction flask, and use the same procedure to transfer TiCl4 solution (20ml) from its vial to the flask. Remember to rinse the syringe as described above.
8. Now turn off the nitrogen tap and introduce a moderate flow of ethylene into the stirred suspension of Ziegler-Natta catalyst.
During the course of the reaction, note any changes in mixture temperature and adjust the supply of ethylene gas so that its supply slightly exceeds demand (i.e. - the exit bubbler should bubble occasionally, but should never be allowed to suck back).
9. When the reaction mixture becomes thick with product, or when the rate of ethylene absorption slackens significantly, the mixture can be hydrolyzed to allow recovery of the product: Turn on the nitrogen and then turn off the ethylene supply.
10.Hydrolyze the reaction mixture by judicious addition of several 2ml aliquots of ethanol. Cool the flask in an ice bath if the reaction becomes too hot. When further addition of ethanol causes no reaction (i.e. heating), suction-filter the reaction mixture in the open and wash the solid several times with ethanol.
11. Clean up glassware,3 rinse with copious ethanol and place in oven for next user.
Procedure - Period 2
Any residual colour in the polyethylene powder, due to catalyst, can usually be removed by standing the solid until the next period in a 1:1 mixture of ethanol and conc. NH3 .
1. Filter and then dry the powder thoroughly, record its weight and measure its melting point.
Along with the usual laboratory write-up (e.g. possible mechanisms of catalysis,4 nature of the Ziegler catalyst,
roles of AlEt3, TiCl4) try to address the following questions in your report:
Questions
1. The stock solutions of AlEt3 and TiCl4 are each 0.12 M in concentration. How many molecules of ethylene were polymerized by each Ti or Al?
2. The Ziegler-Natta catalyst makes "high-density" polyethylene. How does it differ from "low-density" polyethylene in chemical and physical terms?2
3. Why should the petroleum ether medium used in this preparation be olefin-free?
4. What is vermiculite? Why is it suitable for application to spillages in this experiment? (Hint: what material does Aldrich pack its air-sensitive chemicals in; why?).
References and Notes
Animation of polymer formation mechanism
1 Pure TiCl4 is a colourless liquid that fumes vigorously in moist air giving voluminous clouds of white smoke of TiO2 mixed with HCl(g) it is in fact sprayed by light aeroplanes for skywriting. Pure AlEt3 is a colourless liquid that ignites spontaneously in air. However, dilute solutions of it do not usually ignite when exposed to air.
2 Parshall, G. W.; Ittel, S. D. Homogeneous Catalysis; 2nd ed.; John Wiley and Sons, Inc.: New York, 1992; Chapter 4.
3 The removal of the polymer from the glassware during cleanup is generally a problem. Try lots of scrubbing with Comet.