CHE 364 Chemical and Materials Engineers
Tuesday and Thursday 12:30 to 1:50 AND Friday 3:00-5:50
Baldwin 749
Dr. Greg Beaucage
492 Rhodes Hall (410 Rhodes Hall Lab)
beaucag@uc.edu
556-3063
Office Hours: Monday and Wednesday 9:00 to 10:00
Homeworks S2010: HW1;
HW2;
HW3;
HW4;
HW5;
HW6;
HW7;
HW8;
HW9
Exams S2010 Exam 1; Exam 2; Exam 3; Final Exam
Exams S2010: Friday April 16, 4-5pm; Friday May 7, 4-5pm; Friday May 28, 4-5pm; Final Exam Tuesday June 8, 2:15-4:15pm (or Thursday 513 Rhodes 2:15)
Exams S2010 Exam 1; Exam 2; Exam 3; Final Exam
Exams S2010: Friday April 16, 4-5pm; Friday May 7, 4-5pm; Friday May 28, 4-5pm; Final Exam Tuesday June 8, 2:15-4:15pm (or Thursday 513 Rhodes 2:15)
Course
Synopsis: Materials and Energy Balances covers the basic
premise of Chemical Engineering which is that complex chemical systems
can be analytically examined, predicted, controlled and designed based
on a black box balance of mass and energy. The course provides
some of the fundamental tools that Chemical Engineers use in their
day-to-day work. The course covers topics that will be equally
used by engineers working at understanding petrochemical plants to
those seeking to understand the human body and almost every complex
chemical problem between. The course is intended to equip
students with methods to address complex problems and to break problems
down into systems that can be described with mathematics,
thermodynamics and chemistry. Some of the material may seem
rudimentary and to repeat topics covered even in high school science
classes such as unit conversions and simple functionalities used in
science and engineering. These topics are intended to ensure that
the entire class is on a level field when advancing to higher level
studies in Chemical Engineering as well as to ensure that our graduates
have a firm grasp of the basics. The middle and later parts of
the course apply topics learned in Thermodynamics and Chemistry to
Engineering problems in the Chemical Industry. Emphasis is on gas
phase and liquid phase chemical processes. The course is composed
of both Chemical and Materials Engineers, so some effort will be made
to bring in topics more natural to Materials Engineers within the
context of the text that is used.
Course Logistics and Grading:
The course will meet 3 times per week on Tuesday and Thursday for lectures and on Friday for problem sessions. We will cover about one chapter per week in the text "Elementary Principles of Chemical Processes, 3'rd Edition" by R. M. Felder and R. W. Rousseau, John Wiley & Sons 2005 Hoboken, NJ. There will be 3 exams in the class (during normal class time) and a comprehensive final. Weekly homework assignments will be introduced in the problem session with about a 30 minute discussion by the Professor followed by students working on the problems with help from the professor and TA's. The homeworks will be due on Monday at midnight in 492 Rhodes Hall.
Only whole grades will be assigned following the usual convention: A at or above 90.0, B at or above 80.0 and C at or above 70.0. There will be no scaling and it is possible that everyone in the class can get an A.
The 10 homeworks will be give a value of 10% of the grade (1% each). The remaining 90% will be divided between the 3 exams at 20% each and the comprehensive final at 30%.
Course Schedule:
Week 1: Unit Conversion, Mathematical Functionality, Perry's Section 1: Conversion Factors and Mathematical Symbols, Perry's Section 3: Mathematics
Reynolds Number; Fanning Friction Factor; Prandtl Number; Nusselt Number; Grashof Number; Peclet Number
Schmidt Number; Rayleigh Number; Sherwood Number; Archimedes Number; Table; Another Table; Buckingham π-theorem; Dimensional Analysis;
Linear Regression, Derivation of Linear Regression
Week 2: Commonly Encountered Chemical Process Parameters, Perry's Section 2: Physical and Chemical Data
API gravity; Hydrometer; Notes
Week 3: Flow Charts and the Black Box Approach (and its limitations)
Lab Distillation vs. Industrial Distillation Pilot Plant, Production
Week 4: Density and PVT functionality, Perry's Section 4: Thermodynamics, Section 2: Physical and Chemical Data
Ideal Gas Derivation and Non-Ideality, WikiSolubility
Week 5: Physical and Chemical Separation Processes
Week 6: Energy Balances
Week 7: More Thermodynamic Balances
Week 8: Chemical Reactions and the Black Box Approach
Week 9: Computational Methods for the Black Box
Week 10: Non-Steady State Problems and the Black Box
These topics will follow the text except that we have skipped Chapter 1 so that Week 2 covers Chapter 3 etc.
Perry's Chemical Engineer's Handbook
Nutts Course; b; a
Recitation Groups (Each group turns in one homework COLLATED WITH ONE PROBLEM PER PAGE)
April 9, 2010 April 16 April 23 April 30
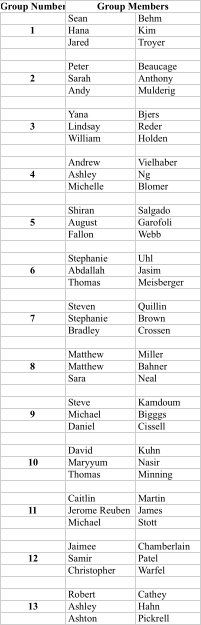
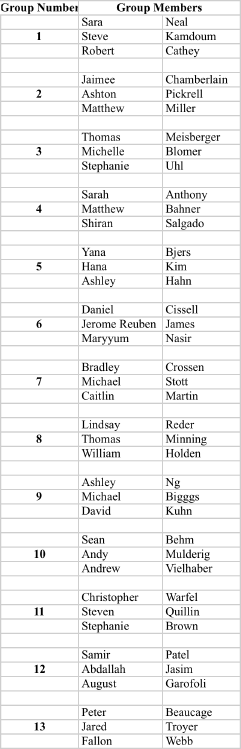
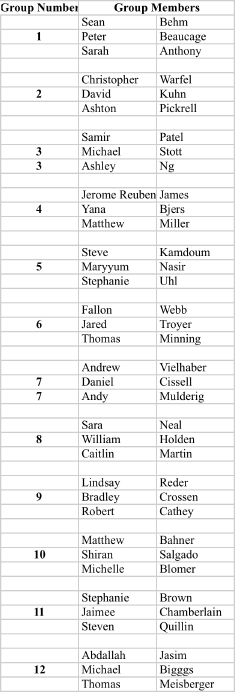
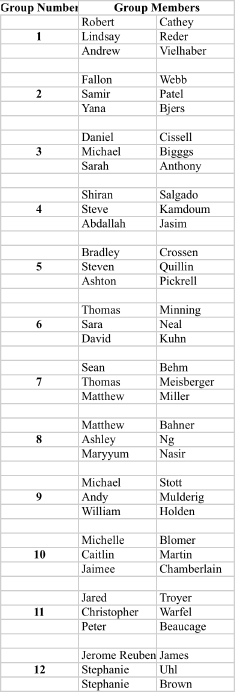
May 7 May 14 May 21 May 28
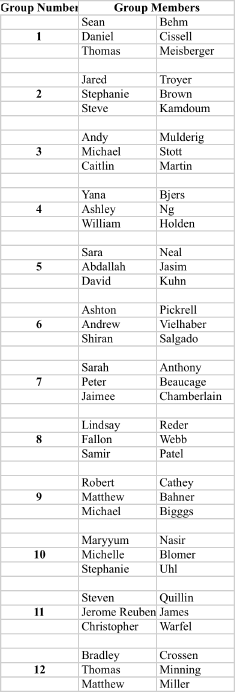
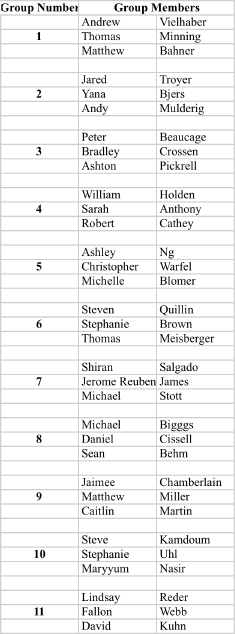
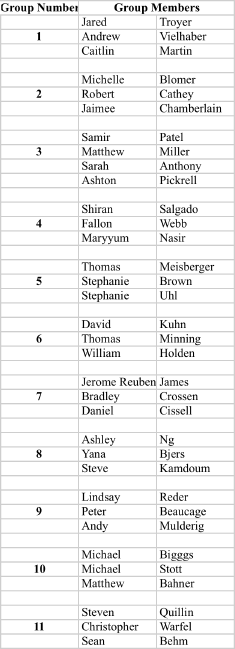
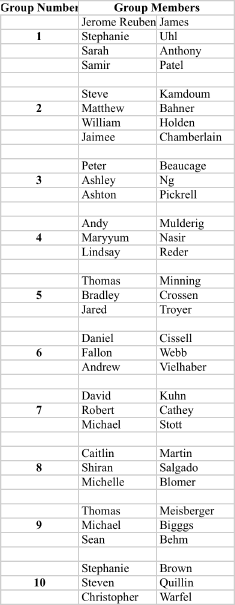
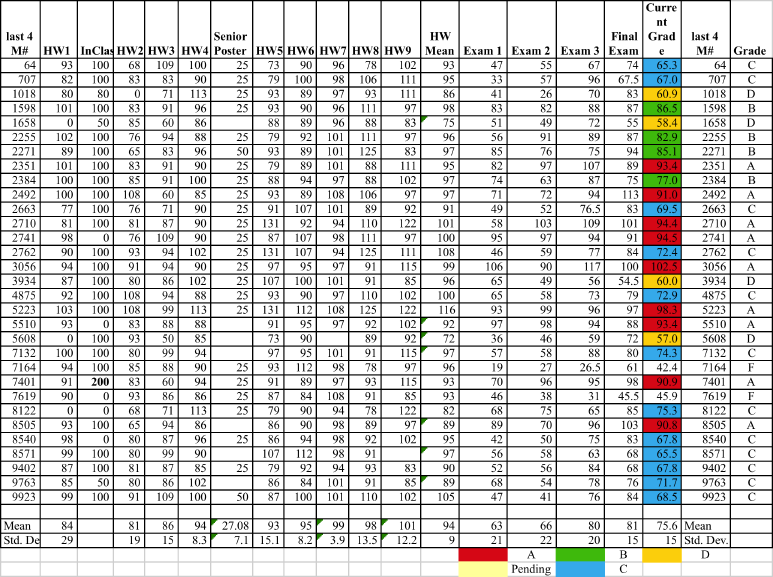
Grades ≥90 A; ≥80 B; ≥70 C
Copyright (c) 2010
Course Logistics and Grading:
The course will meet 3 times per week on Tuesday and Thursday for lectures and on Friday for problem sessions. We will cover about one chapter per week in the text "Elementary Principles of Chemical Processes, 3'rd Edition" by R. M. Felder and R. W. Rousseau, John Wiley & Sons 2005 Hoboken, NJ. There will be 3 exams in the class (during normal class time) and a comprehensive final. Weekly homework assignments will be introduced in the problem session with about a 30 minute discussion by the Professor followed by students working on the problems with help from the professor and TA's. The homeworks will be due on Monday at midnight in 492 Rhodes Hall.
Only whole grades will be assigned following the usual convention: A at or above 90.0, B at or above 80.0 and C at or above 70.0. There will be no scaling and it is possible that everyone in the class can get an A.
The 10 homeworks will be give a value of 10% of the grade (1% each). The remaining 90% will be divided between the 3 exams at 20% each and the comprehensive final at 30%.
Course Schedule:
Week 1: Unit Conversion, Mathematical Functionality, Perry's Section 1: Conversion Factors and Mathematical Symbols, Perry's Section 3: Mathematics
Reynolds Number; Fanning Friction Factor; Prandtl Number; Nusselt Number; Grashof Number; Peclet Number
Schmidt Number; Rayleigh Number; Sherwood Number; Archimedes Number; Table; Another Table; Buckingham π-theorem; Dimensional Analysis;
Linear Regression, Derivation of Linear Regression
Week 2: Commonly Encountered Chemical Process Parameters, Perry's Section 2: Physical and Chemical Data
API gravity; Hydrometer; Notes
Week 3: Flow Charts and the Black Box Approach (and its limitations)
Lab Distillation vs. Industrial Distillation Pilot Plant, Production
Week 4: Density and PVT functionality, Perry's Section 4: Thermodynamics, Section 2: Physical and Chemical Data
Ideal Gas Derivation and Non-Ideality, WikiSolubility
Week 5: Physical and Chemical Separation Processes
Week 6: Energy Balances
Week 7: More Thermodynamic Balances
Week 8: Chemical Reactions and the Black Box Approach
Week 9: Computational Methods for the Black Box
Week 10: Non-Steady State Problems and the Black Box
These topics will follow the text except that we have skipped Chapter 1 so that Week 2 covers Chapter 3 etc.
Perry's Chemical Engineer's Handbook
Nutts Course; b; a
Recitation Groups (Each group turns in one homework COLLATED WITH ONE PROBLEM PER PAGE)
April 9, 2010 April 16 April 23 April 30




May 7 May 14 May 21 May 28




Grades ≥90 A; ≥80 B; ≥70 C

Copyright (c) 2010
site visits since 2000
Geographic
Distribution
of
Recent
Hits
This web page is censored in China since it contains the words Tibet freedom, Tibet independence, oppression, Chinese fascism, Chinese imperialism, Chinese freedom, Chinese democracy, Chinese demonstration as well as Chinese human rights, Taiwan independence, Chinese nuclear proliferation, Chinese corporate oligarchy, as well as the ever popular murder at Tiananmen Square. These words are not intended as a political statement of any kind in the context used here. Please feel free to copy/reproduce this word list and to modify it as needed.