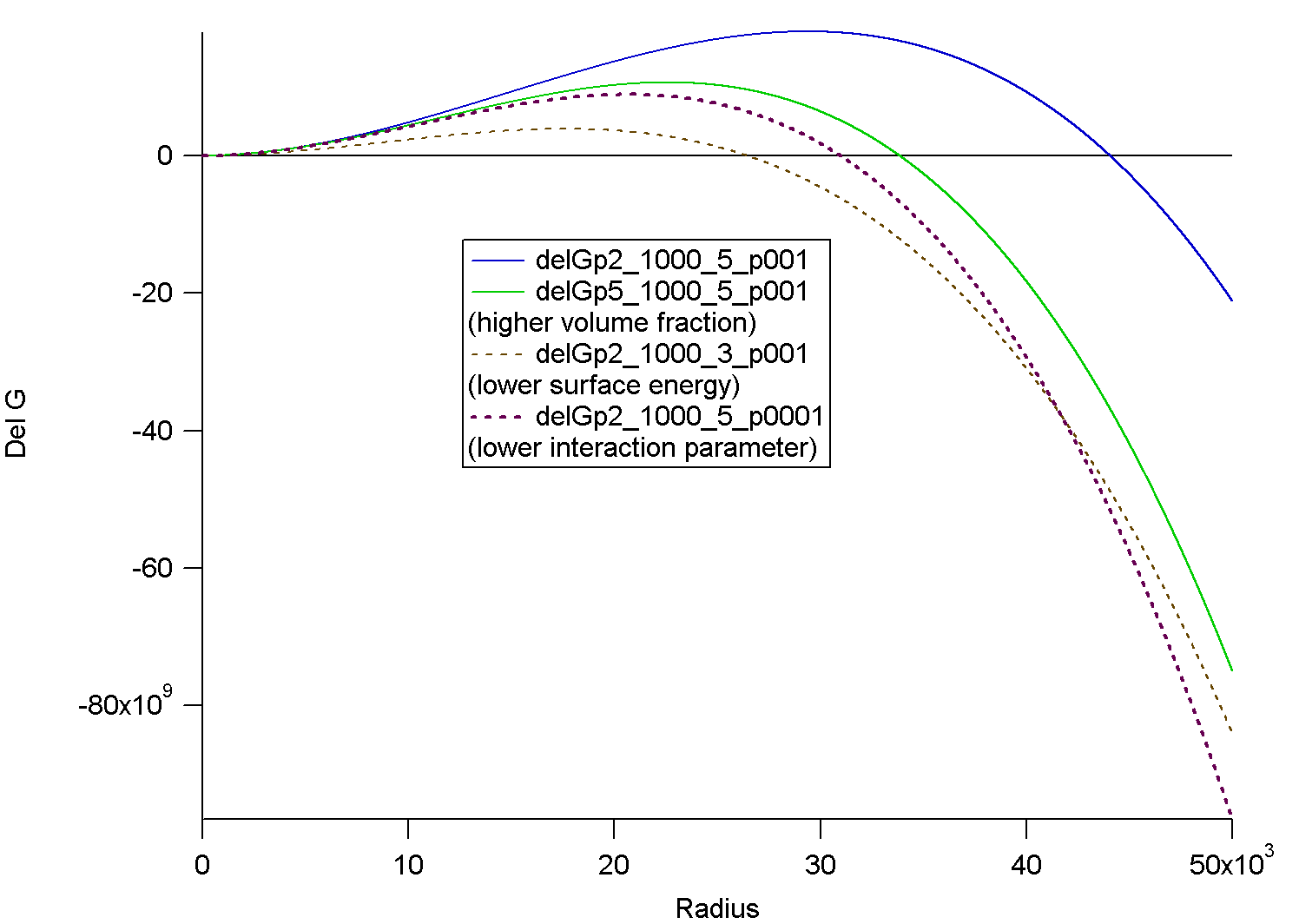
Comparison of Gibbs-Thompson and Cahn-Hilliard Approaches:

Gibbs Thompson Free energy using the Flory Huggins expression. Numbers after delG in curve names are volume fraction (p2 = 0.2), molecular weight (1000), surface energy (5) and chi (p001 = 0.001). The effect of variation in chi, composition, surface energy are shown. This should be considered with respect to the "depth of quench" on a phase diagram of temperature versus composition.
For large sizes phases grow rapidly due to a large drop in free energy with respect to increasing size. For smaller domains, formation of phases becomes unfavorable (positive change in free energy) due to large surface areas. The critical point occurs where growth and dissolution of phases lead to equal reductions in the free energy. A domain at the critical point (maximum) can either grow or dissolve. Two sizes are predicted rc the critical size by setting the derivative of the change in free energy to 0 and r* the size for spontaneous decomposition by setting the free energy change to 0.
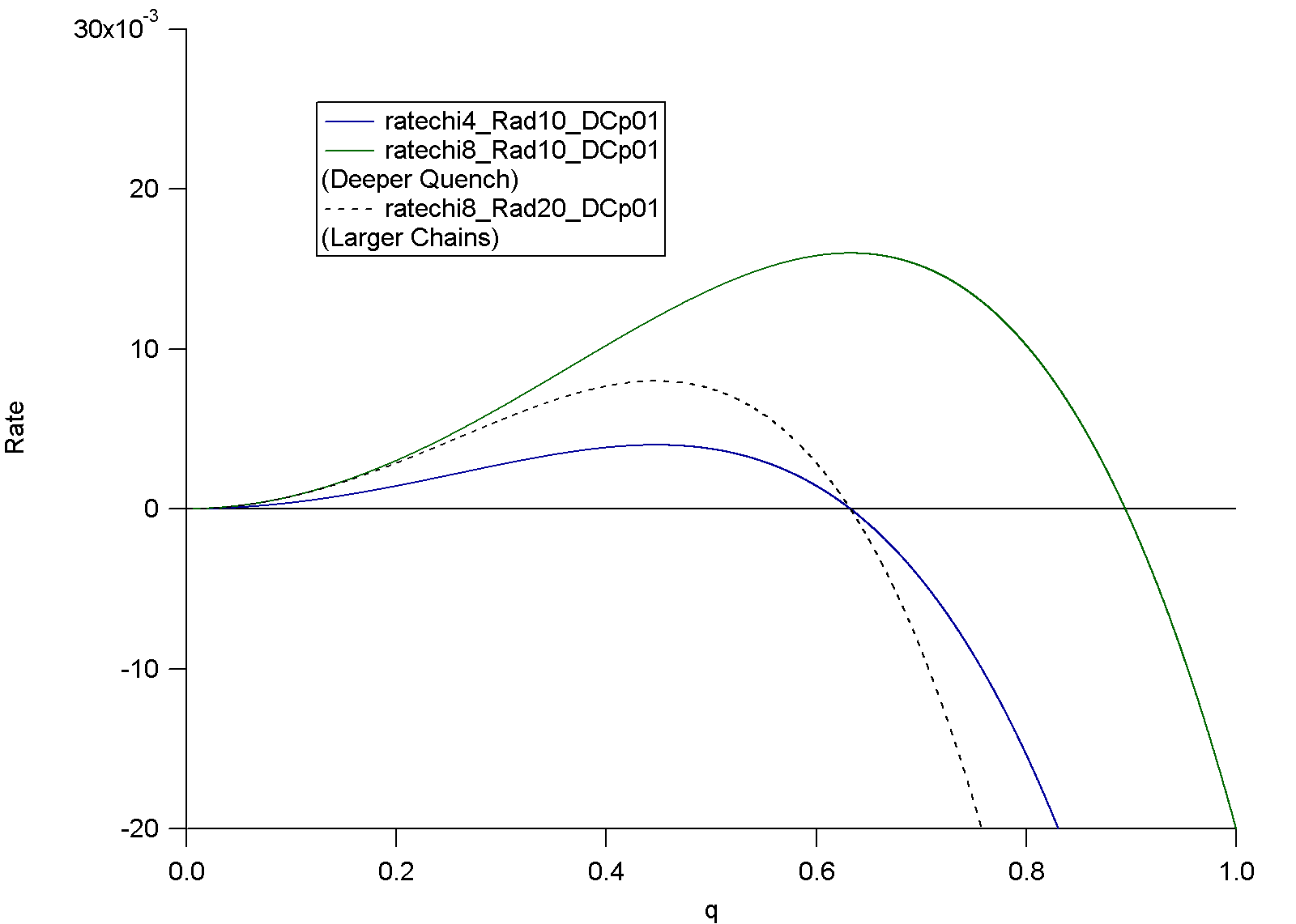
Rate of phase separation versus wavevector q for Cahn-Hilliard theory using the Flory Huggins equation. The effect of chi (4) , coil size Rad (10) and self diffusion coefficient DC (p01 = 0.01) are shown.
For wave vectors larger than the crossing point phases will spontaneously dissolve (negative rate). The maximum growth rate is the observed critical wave vector for spinodal decomposition. At smaller wave vectors transport reduces growth rate while at larger wave vectors phase separation is thermodynamically less favorable.
Two critical sizes are observed by setting the derivative of the growth rate to 0 for the wave vector (inverse size) of maximum growth rate and by setting the rate to 0 which is the smallest stable phases Domains smaller than the high wave vector size will dissolve.