Osmotic Pressure
and the Virial Expansion:
Kinetic Theory of Gasses:
The story of osmotic pressure begins in an unlikely place, the kinetic theory of gasses. For a gas composed of particles with no volume but with a mass that allows them to reach thermal equilibrium with their environment, the pressure, P, is proportional to the kinetic energy, kT, and the concentration of gas molecules, n/V. This assumes there are no collisions between gas molecules, i.e. no interaction, and that the gas particles move by Brownian motion.
If the constraints are relaxed and the gas atoms are allowed to interact to some extent, that is they have volume, then the motion is not totally Brownian. We can consider interactions in orders, binary interactions, which are proportional to the volume fraction squared, ternary interaction, volume cubed and so forth. Such an expansion of the ideal gas law is called a virial expansion and the gas law becomes,
![]() Non-Ideal
Gas
Non-Ideal
Gas
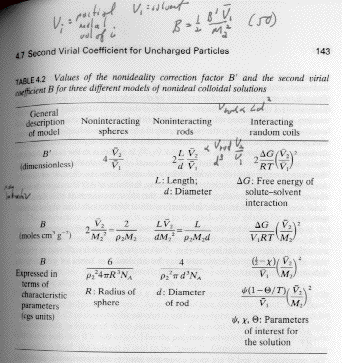
The second virial coefficient describes binary interactions. Remember that the Flory Lattice theory also includes only binary interactions. The following table is from Paul Heimenz, "Principles of Colloid and Surface Chemistry" first edition (also probably in the second edition) that describes how different fields have interpreted the second virial coefficient. B is a virial coefficient for a mass concentration rather than a molar concentration, but the two second virial coefficients scale. The last column is the interpretation for polymer coils, the others pertain to other types of colloidal particles, such as rod like viruses (Tobacco Mosaic Virus) and spherical colloids such as colloidal silica. It should be clear that there can be many interpretations of the virial expansion, the simples t being a geometric interpretation, first two columns in Heimenz's table. For our purposes, the second virial coefficient will be represented by binary interactions in a polymer solution that are associated with excluded volume (non-interaction spheres of Heimenz's table).
The first virial coefficient, A1, reflects some mechanism of amplifying the effective number of particles or reducing the effective volume. This is of importance to polymers since the number of particles could be considered from the perspective of a mer or from the perspective of a chain or from the perspective of a concentration blob. The higher order virial coefficient reflect higher order interactions or geometric features such as the curvature of the particle or surface features.
Colloidal Solutions and Osmotic Pressure:
For a colloidal solution the suspended particles move by Brownian motion and at equilibrium each carry kT kinetic energy which can be deposited on the walls of the vessel in which they are contained. This is identical to the kinetic theory of gasses and the same analysis is used to predict the excess pressure, p, associated with n molecules of colloid in a volume V, where the
molar concentration is given by, x = n/V. If a solvent and a solution are in contact through a semi-permeable membrane that allows only solvent to pass through, then the excess pressure, p, can be measured by the difference in height of the two chambers since the chemical potential, and associated pressure of the solvent are equal on the two sides of the membrane at equilibrium. A virial expansion can be used with the same assumptions involved in the non-ideal gas kinetic theory,
![]() Colloid
Particles
Colloid
Particles
If g grams of polymer are added to a total volume V of solution, and the molecular weight is unknown, x can be calculated from g/(M0NA), but this is the number concentration of monomers. The concentration must be reduced by the number of monomers in the chain, N, so the virial expansion becomes,
![]() Flory-Huggins
Polymer
Flory-Huggins
Polymer
For large molecular weights, N>>>1, and high concentrations the first term is 0 and the osmotic pressure scales with the volume fraction squared. At low concentrations, x<<1, a constant value is obtained for p/(kTx) that is proportional to the inverse of the molecular weight. The Flory Huggins expression relies on the same assumptions as the kinetic theory of non-ideal gasses with binary enthalpic interactions or equivalently, with a non-zero value for the excluded volume. The function reduces to the ideal case when c => 1/2. Then the first term in the expansion reflects the ideal conditions of particles (polymer coils) in a volume with no interaction and with total energy kT for the coil. The scaling features of the coil do not matter as long as it has a roughly spherical shape and can be deal with as a unit. The Gaussian assumption of Flory-Huggins theory is embodied in the higher order terms, particularly the scaling of p with f2. Then it is not surprising that the ideal component has been repeatedly verified experimentally, while the experimental evidence deviates from a f2 scaling law. The true scaling law for osmotic pressure at moderate concentrations, above the overlap concentration, was explained in the 1970's by des Cloiseaux in terms of the concentration blob model. The result of this analysis is a scaling law of p~(f/f*)9/4 which has been repeatedly verified by experiment and is discussed in the next section.