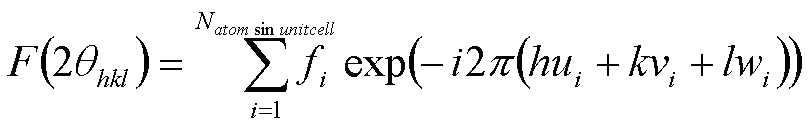 (1).
(1).Construction of a scattered intensity.
In calculating the intensity scattered (diffracted) from a crystalline phase in the irradiated volume of a sample (this is the footprint of the beam on the sample times the thickness for a transmission measurement) we consider a model based on decomposition of the structure into levels of structure composed of structural elements. The irradiated volume is composed of Ngrain large grains (compared to the wavelength of x-rays) each containing on average Nunit cell repeat unit cells of the crystalline structure. The scattering from the irradiated volume is then the scattering from a unit cell, F2(2q), time the number of unit cells in the irradiated volume, NgrainNunit cell. Here we just sum the intensities from unit cells rather than considering the phase difference between unit cells since for every two unit cells that could give rise to a given constructive interference peak at an arbitrary d-spacing of the separation distance between two unit cells, there is always another unit cell at exactly 1/2 this distance that gives rise to completely desctuctive interference. This is true from the unit cell size to the grain size so we do not expect scattered intensity for angles between the smallest size for unit cell diffraction to angles associated with the grain size. The grains could have interference that would give rise to scattering but they are much to large to see at angles observed in diffraction.
The scattering from a unit cell, F2(2q), is calculated from the structure factor, F(2q). The structure factor accounts for each of the atoms in a unit cell by considering the scattering from the atom if were isolated, the atomic form factor, and the interference that results from the arrangement of the atoms as accounted by a summation of terms with amplitude, f, and phase, exp(-i2p(hu+kv+lw)), where hkl are the Miller indices for the plane of interest ((hkl) yields 2q through the d-spacing of these planes and Bragg's Law, for example, for cubic 1/d2 = (h2+k2+l2)/a2 and d = l/(2sinq)),
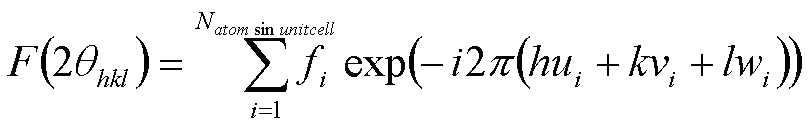 (1).
(1).
The form factor, f, can be obtained from tabulated values for atomic form factors or from functions available on the internet from the International Crystallographic Association. The atomic form factor accounts for the number of electrons in an atom, or atomic number Z, and for the interference between electrons in an atom. Since the electrons do not have a regular arrangement, the interference pattern can not be easily calculated so tabulated values that are derived from experimental observations are used for the atomic form factor. Then we consider the interference between atoms in F and the contribution from an isolated atom in f. Similarly, we consider the interference between electrons in f and the contribution from a single, free electron in the Thompson Factor. A single free electron is an isolated electron that can not sense other electrons. The Thompson factor is then the base unit for the calculation of diffracted intensity, that is it is the brick form which the wall and the building of diffracted intensity are constructed. The Thompson factor allows for a calculation of absolute intensity. It is also responsible for the effect of polarization on the final diffracted or scattered radiation and it is from the Thompson factor that the Lorentz Polarization Factor is calculated. Just as a single isolated unit cell and a single isolated atom are not present in the irradiated volume, though these are used to calculate the diffracted intensity, a single isolated electron is also not present in the irradiated volume. The single isolated electron is just used as a calculation tool since it is more general to consider the bricks of the wall as isolated units that contribute to the diffracted intensity.
Finally, it is often convenient to consider the scattering element associated with the atomic form factor, f, in a broader sense as relating to any substructural element that repeats on the crystallographic lattice. For instance, in NaCl we consider the repeat unit to be two atoms, Na at [000] and Cl at [1/2,1/2,1/2]. Then the scattering is calculated from the interference between Na and Cl in the repeat unit, (fNa + fCl exp(-ip(h+k+l))), times the interference associated with the FCC structure (1+exp(-ip(h+k))+exp(-ip(h+l))+exp(-ip(k+l))). For organic and bio-systems the unit cell structure is often extremely simple while the repeat unit is of high complexity. For example in protein diffraction we consider a repeating arrangement of protein molecules in perfect alignment along a crystal lattice. It is the scattering (diffraction) from within the individual unit cell sites that is of interest since this yields the native structure of the protein molecule. The analysis is based on the approach taken with NaCl but with a much more complicated molecule with thousands or tens of thousands of atoms. Such diffraction patterns often can only be solved (resolution of structure) using supercomputers.