

This Web page is supported by the National Science Foundation through grant CBET 0626063 to Beaucage.
Laboratory Module for Production of Nanomaterials in Flames: Maghematite Nanoparticles for Ferrofluids
Introduction:
The purpose of this module will be
In the lab a simple diffusion flame will be used to produce maghemite nanoparticles from an oil lamp loaded with a propanol/ferrocene fuel blend. Particles are collected through a combination of thermophoresis (eqns.) and by magnetism using a coffee can filled with cold water and magnets (Figure 1). These particles will be used to produce a ferrofluid in class. The nano-particles will be examined using transmission electron microscopy (TEM) and small-angle x-ray scattering (SAXS) at the University of Cincinnati. Discussion will be made of the use of maghemite particles to separate arsenic from drinking water by absorption and magnetic separation [1].
Required Materials:
For Ferrofluid:
tetramethylamoniumhydroxide
We have performed this experiment with no hood or with a hood. The need for a hood depends on the time of combustion, room ventilation, size of the oil lamp and the like. The flame produces CO2 and some uncollected nanoparticles.
Background:
Nanomaterials are ordinary materials produced in extremely small pieces. Generally, we consider that the intensive properties such as density and reactivity and magnetic susceptibility of a material do not depend on size. This is generally true to sizes of about 100 nm. Below these sizes enhancement and dimunition of certain intensive properties. Extensive properties such as the specific surface area (S/V ~ 1/D) change directly with particle size. The engineering properties of a material for a particular application can be tuned using particle size and for many applications particles on the nanoscale or nanoparticles have special advantages due to a combination of intensive and extensive properties. For example, the magnetic susceptibility of ferromagnetic materials is known to decay at sizes on the order of 10 nm and smaller. This can result in a loss in the ability of the material to be separated from a fluid suspension or a loss in ferrofluid behavior for extremely small suspensions. To some extent this loss in magnetic properties can be mitigated by aggregation of these extremely small particles.
While the extensive property of magnetic susceptibility is known to decay, the specific surface area increase with reduction in particle size leads to dramatically enhanced ability per gram of material to carry out surface reactions and associations. For maghemite particles produced in a flame the enhancement in intensive properties dramatically increases the usefulness of these iron oxides for adsorption of heavy metals such as arsenic. By using particles in an intermediate nanosize range an optimized nanoparticle system can be designed to use magnetic separation of arsenic from drinking water [1].



(a)
(b)




(c)
(d)
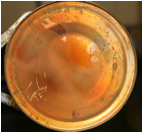
Figure 1.
a) Simple oil lamp burner with ethanol/ferrocene
solution as fuel for high school teaching module. b) Thermophoretic
and magnetic collection of nanoparticles
using a coffee can filled with cold water and magnets for high school
teaching module. c) Hematite nanopowder
from flame spray pyrolysis (figure 1b) at
UC. d)
Magnetic separation of hematite nanoparticles
from water at UC as a route to heavy metal remediation from drinking
water.
Experimental:
Introduction: We have developed a project based on the theory that Iron Oxide (Fe2O3) nanoparticles can be obtained through the combustion of ferrocene in solution. The solution to be burned can be made using ethanol, propanol, acetate, or lamp oil as a precursor. Our experimentation showed ethanol to be the best choice of solvent, because it yields the most product, is easiest to control while burning, and is relatively obtainable. The ratios of ferrorcene to precursor we experimented with were 1:1, 2:1, 4:1, and 10:1. 4:1 one was found to be the best for getting all of the solvent to dissolve and still yielding a collectable amount of product after combustion. A 10:1 ratio yielded the greatest amount product, but the solution needed to be heated in order for all of the ferrocene to completely dissociate. Combustion was done using a regular oil lamp. We found that using the base of a smaller lamp and the top from a larger lamp yielded optimal results. The small base worked out well, because it held between 80 and 160 ml of solution, which is a quantity that we often used. The top from a large lamp worked, because it had a longer, thicker wick. This combination allowed the wick to stay immersed and saturated with solution during the entire burning process, because the wick was long enough to reach the bottom of the base. Various methods were used for collection. The greatest amount of product could be collected using a filtering system that was attached to a vacuum. A sheet of glass filter paper was suspended over the flame. The particles came off the flame and were sucked onto the filter paper by the vacuum. After combustion, the sample can then be scraped off of the filter paper and into vials. Another method to collect the product is using the magnetic qualities of ferrocene. This method was not as effective quantitively, but involving the magnetic property in the experiment makes it more interesting. During out experimentation, a metal surface with magnets on top was placed over the flame. The nanoparticles were attracted to the magnets, and stuck to the surface. A metal container (like a coffee can) filled with an ice water bath seemed to work best. If the magnets are placed inside the can, the bottom will attract the nanoparticles while the ice bath keeps the entire system from overheating. The product can then be collected by scraping it off of the metal with something soft, like a small piece of filter paper.
FerroFluid Part (Based on UCLA Instructions.pdf, Teacher Manual from UCLA.doc):
References:
1) Low-Field Magnetic Separation of Monodisperse Fe3O4 Nanocrystals. Yavuz CT, Mayo JT, Yu WW, Prakash A, Falkner JC, Yean S, Cong L, Shipley HJ, Kan A, Tomson M, Natelson D, Calvin VL Science 314 964-967 (2006).
2) Engines and nanoparticles: a review. Kittelson DB J. Aerosol. Sci. 29 575-588 (1998).